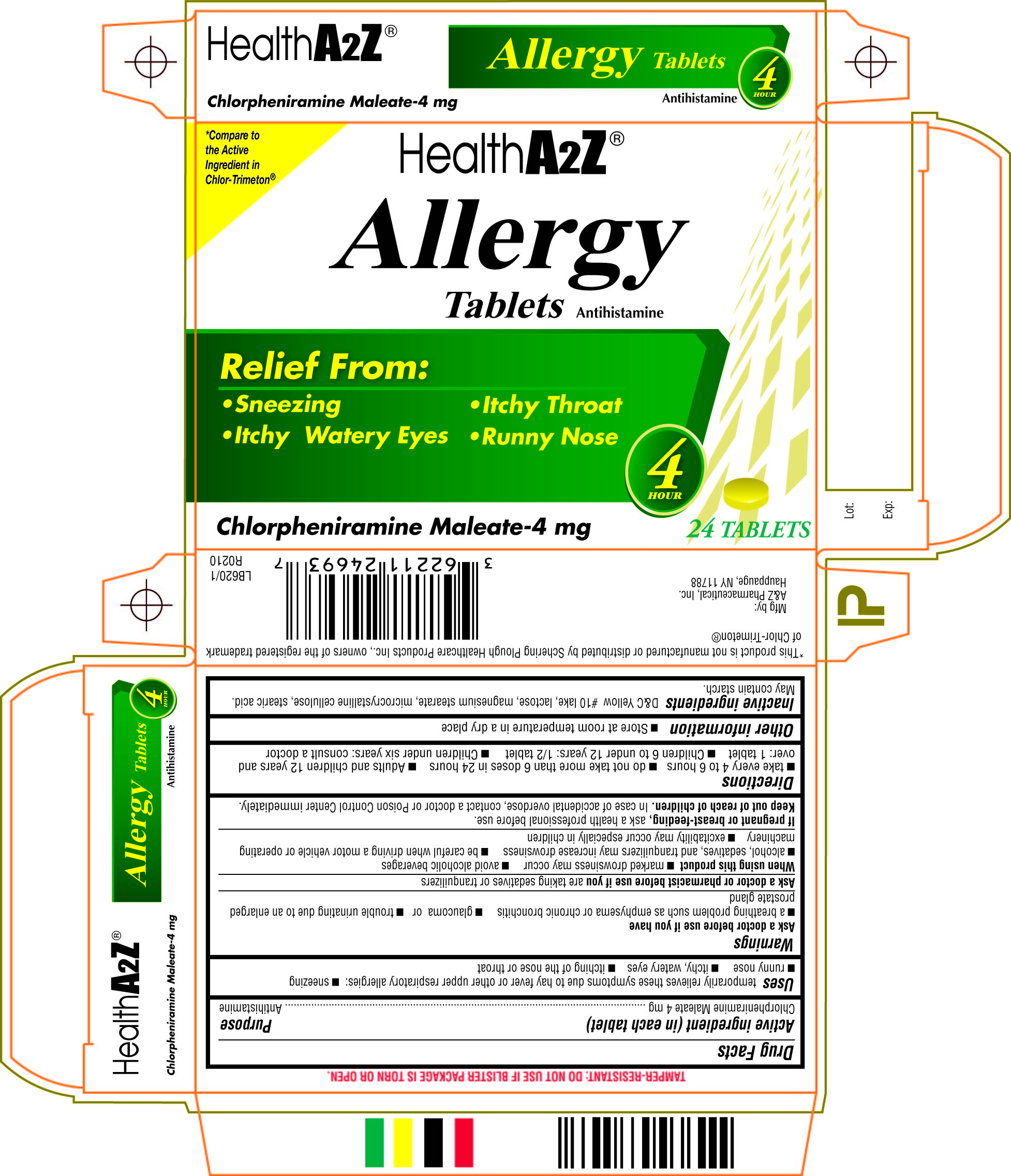
Chlorpheniramine Tablets Prescribing Information
Package insert / product label
Generic name: chlorpheniramine maleate
Dosage form: tablet
Drug class: Antihistamines
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Indications and Usage for Chlorpheniramine Tablets
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: ■ sneezing ■ runny nose ■ itchy, watery eyes ■ itching of the nose or throat
Warnings
Ask a doctor before use if you have
■ a breathing problem such as emphysema or chronic bronchitis, ■ glaucoma or ■ trouble urinating due to an enlarged prostate gland
When using this product
■ marked drowsiness may occur ■ avoid alcoholic beverages ■ alcohol, sedatives, and tranquilizers may increase drowsiness ■ be careful when driving a motor vehicle or operating machinery ■ excitability may occur especially in children
Keep out of reach of children.
In case of accidental overdose, contact a doctor or Poison Control Center immediately.
Directions
■ take every 4 to 6 hours ■ do not take more than 6 doses in 24 hours ■ adults and children 12 years and over: 1 tablet ■ children 6 to under 12 years: 1/2 tablet ■ children under 6 years: consult a doctor
| CHLORPHENIRAMINE MALEATE
chlorpheniramine maleate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - A&Z Pharmaceutical, Inc. (926820705) |
| Registrant - A&Z Pharmaceutical, Inc. (926820705) |
Frequently asked questions
More about chlorpheniramine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (54)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antihistamines
- Breastfeeding